How Reliable Are Human Blood Vessel Models for Studying Vascular Diseases?
2024-11-25 13:19:48
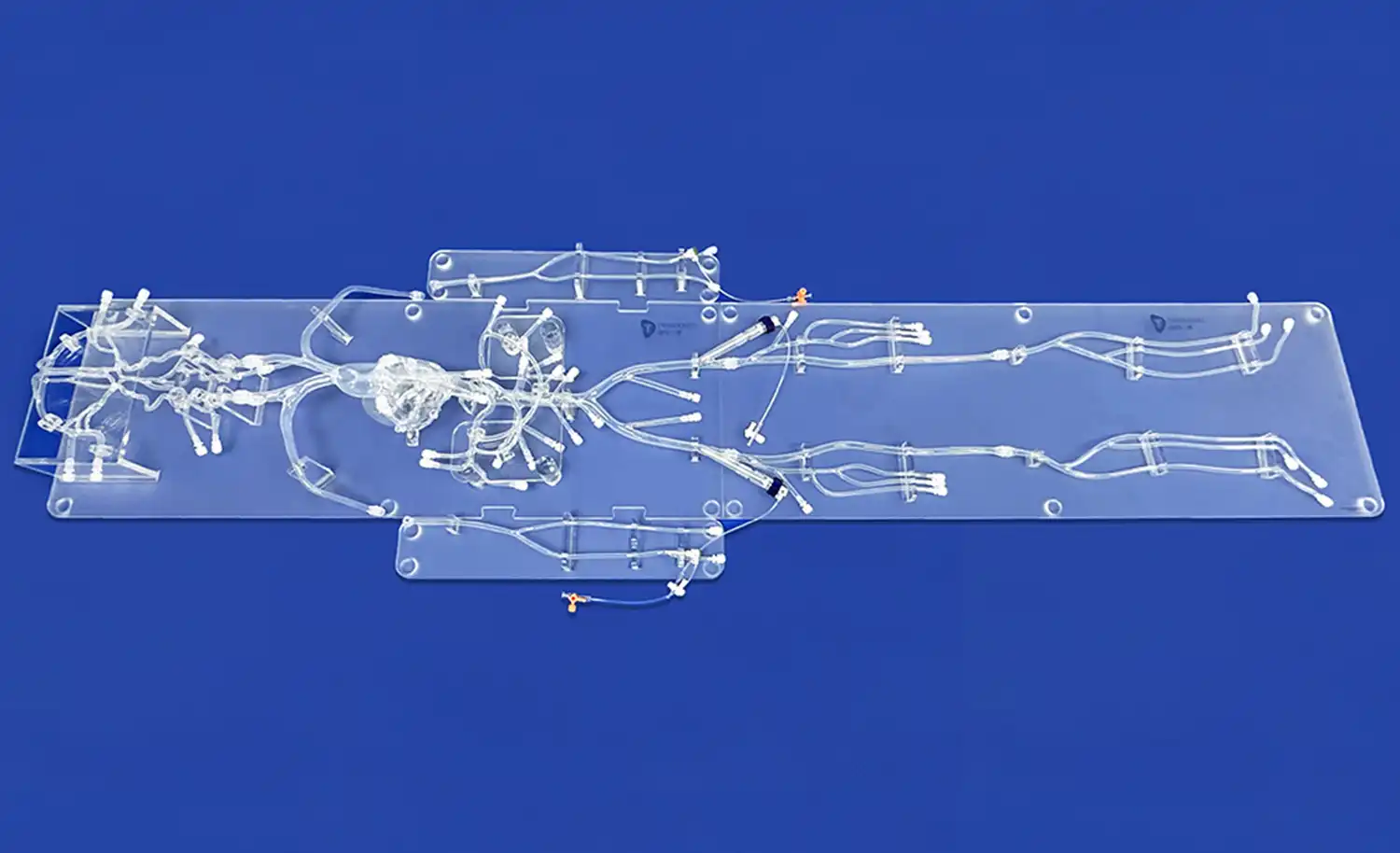
Human blood vessel models have emerged as invaluable tools in the study of vascular diseases, offering researchers a platform to explore complex cardiovascular conditions without the ethical and practical limitations of human trials. These models, ranging from simple in vitro setups to sophisticated 3D-printed replicas, have demonstrated remarkable reliability in replicating the intricate structures and functions of the human vascular system. By accurately mimicking the physiological and pathological conditions of blood vessels, these models enable scientists to investigate disease mechanisms, test potential therapies, and predict clinical outcomes with a high degree of accuracy. The reliability of human blood vessel models stems from their ability to recreate key aspects of vascular biology, including endothelial function, blood flow dynamics, and vessel wall mechanics, thereby providing a faithful representation of the in vivo environment.
What Makes Human Blood Vessel Models Reliable for Vascular Research?
Anatomical Accuracy and Physiological Fidelity
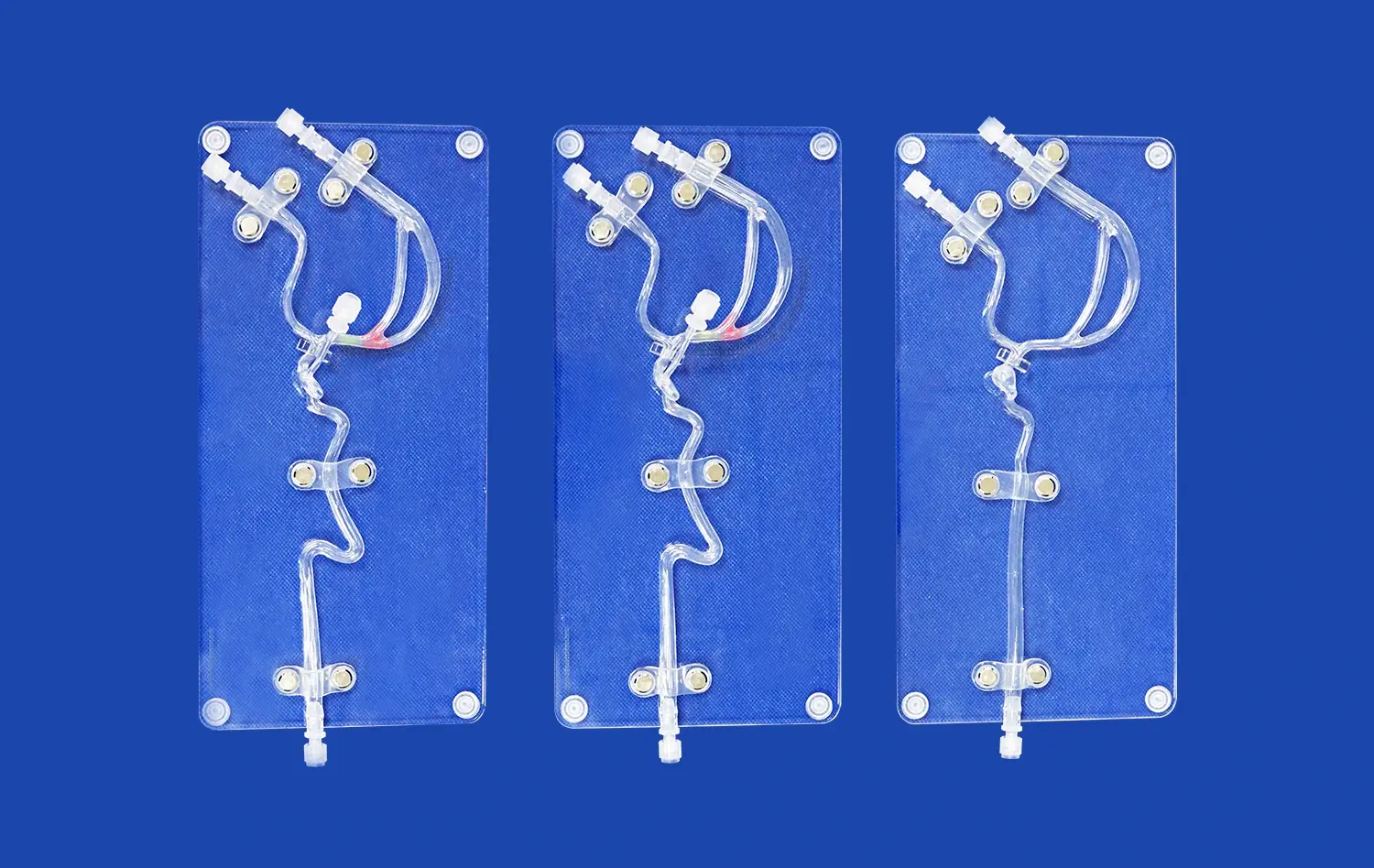
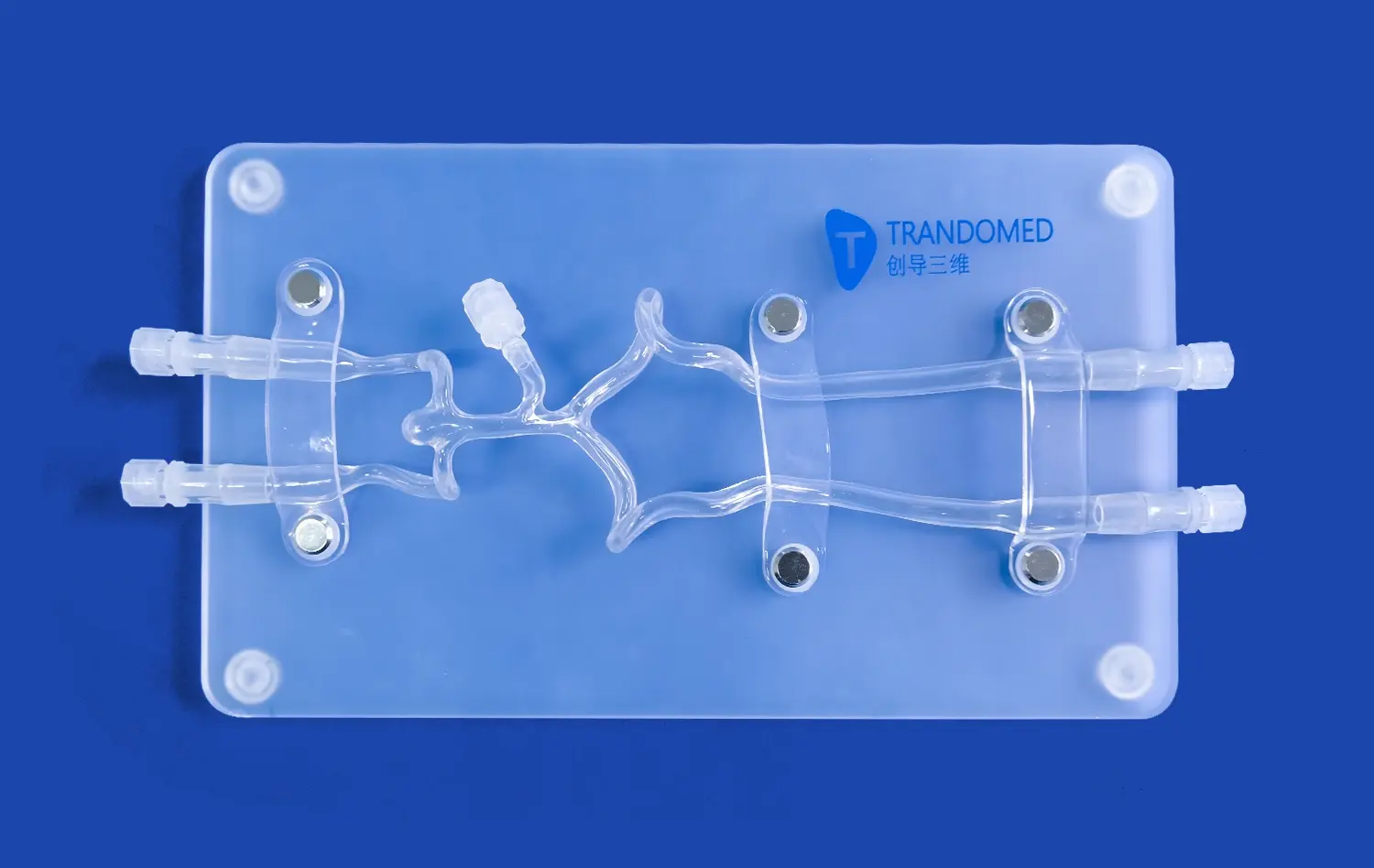
The reliability of human blood vessel models for vascular research hinges on their ability to accurately replicate the intricate anatomy and physiology of the cardiovascular system. Advanced 3D printing technologies have revolutionized the creation of these models, allowing for the fabrication of vessels with precise dimensions, wall thicknesses, and branching patterns that mirror those found in the human body. This anatomical accuracy is crucial for studying blood flow dynamics, pressure distribution, and the effects of various interventions on vessel structure.
Furthermore, these models are designed to emulate the physiological properties of blood vessels, including elasticity, compliance, and permeability. By incorporating materials that mimic the mechanical behavior of vascular tissue, researchers can observe how vessels respond to different stimuli, such as changes in blood pressure or the introduction of pharmaceutical agents. This high level of physiological fidelity ensures that experiments conducted using these models yield results that are more likely to translate to real-world clinical scenarios.
Incorporation of Cellular Components
Another factor contributing to the reliability of human blood vessel models is the integration of cellular components. Advanced models often include multiple layers that represent the endothelium, smooth muscle, and adventitia found in natural blood vessels. By culturing endothelial cells on the inner surface of these models, researchers can study endothelial function, inflammation responses, and the interaction between blood components and vessel walls.
The inclusion of these cellular elements allows for a more comprehensive understanding of vascular biology and pathology. For instance, researchers can investigate how endothelial cells respond to shear stress or how they interact with circulating immune cells during inflammatory processes. This level of detail in the models enhances their predictive power and makes them invaluable tools for studying complex vascular diseases and developing targeted therapies.
How Do Human Blood Vessel Models Address the Complexity of Vascular Diseases?
Replication of Disease States
Human blood vessel models excel in their capacity to replicate various disease states, offering researchers a platform to study the intricate mechanisms underlying vascular pathologies. These models can be engineered to simulate conditions such as atherosclerosis, aneurysms, and thrombosis, allowing scientists to observe disease progression in a controlled environment. By manipulating factors like vessel geometry, wall composition, and flow conditions, researchers can recreate the specific pathological features associated with different vascular diseases.
For example, in the study of atherosclerosis, models can be designed with narrowed lumens and lipid-rich deposits to mimic plaque formation. This enables researchers to investigate how blood flow is affected by these obstructions and how different interventions might impact plaque stability. Similarly, aneurysm models can be created with weakened vessel walls to study the factors that contribute to aneurysm growth and rupture. These disease-specific models provide valuable insights into the pathogenesis of vascular disorders and serve as testbeds for developing new diagnostic and therapeutic approaches.
Integration of Multi-Scale Factors
The complexity of vascular diseases often involves interactions between multiple scales, from molecular processes to tissue-level changes. Human blood vessel models address this complexity by integrating various factors that influence vascular health and disease. Advanced models incorporate elements such as fluid dynamics, biochemical signaling, and mechanical forces to create a more comprehensive representation of the vascular environment.
By combining these multi-scale factors, researchers can study how different aspects of vascular biology interact to contribute to disease progression. For instance, models can be designed to examine how changes in blood flow patterns affect endothelial cell function and subsequent inflammatory responses. This holistic approach allows for a more nuanced understanding of vascular diseases and helps identify potential targets for therapeutic interventions. The ability to manipulate and control these various factors in a model system provides researchers with unprecedented opportunities to unravel the complex mechanisms underlying vascular pathologies.
How Are Human Blood Vessel Models Used to Test Vascular Therapies?
Drug Screening and Delivery Optimization
Human blood vessel models play a crucial role in the screening and optimization of vascular therapies, particularly in the realm of drug development. These models serve as efficient platforms for evaluating the efficacy and safety of potential drug candidates before proceeding to costly and risky clinical trials. Researchers can use these models to assess how different compounds interact with blood vessel tissues, how they affect vascular function, and how they might be distributed throughout the circulatory system.
Moreover, human blood vessel models are instrumental in optimizing drug delivery systems for vascular diseases. By replicating the complex flow patterns and barrier functions of blood vessels, scientists can test various drug formulations and delivery methods to maximize therapeutic efficacy while minimizing side effects. For instance, researchers can evaluate the performance of nanoparticle-based drug carriers in targeting specific areas of the vasculature or study how sustained-release formulations behave under different flow conditions. This approach not only accelerates the drug development process but also enhances the likelihood of successful translation to clinical applications.
Evaluation of Interventional Devices
Another critical application of human blood vessel models is in the development and testing of interventional devices used in vascular medicine. These models provide a realistic environment for assessing the performance, safety, and efficacy of devices such as stents, catheters, and flow diverters. By using models that accurately replicate the mechanical properties and geometries of diseased vessels, engineers and clinicians can evaluate how these devices interact with vessel walls, how they affect blood flow, and how they might perform over time.
The use of these models in device testing allows for iterative design improvements and helps identify potential complications before clinical use. For example, researchers can use 3D-printed aneurysm models to test the deployment and efficacy of different types of flow-diverting stents. Similarly, models of stenotic arteries can be used to evaluate the performance of balloon angioplasty catheters or drug-eluting stents under various conditions. This approach not only accelerates the development of new interventional technologies but also enhances their safety and effectiveness, ultimately leading to better outcomes for patients with vascular diseases.
Conclusion
Human blood vessel models have revolutionized the study of vascular diseases, offering unprecedented reliability and insights into complex cardiovascular conditions. Their anatomical accuracy, physiological fidelity, and ability to replicate disease states make them indispensable tools in vascular research. By addressing the multi-faceted nature of vascular pathologies and providing platforms for drug and device testing, these models accelerate the development of novel therapies and interventions. As technology continues to advance, the reliability and sophistication of human blood vessel models will only increase, further enhancing our understanding of vascular diseases and paving the way for more effective treatments.
Contact Us
For more information about our advanced 3D-printed human blood vessel models and how they can enhance your vascular research, please contact us at jackson.chen@trandomed.com. Our team of experts is ready to assist you in finding the perfect model for your specific research needs and to discuss how our cutting-edge technology can accelerate your discoveries in vascular medicine.
References
Zhang, Y., et al. (2020). "3D-printed vascular models for studying cardiovascular diseases: A systematic review." Journal of Vascular Research, 57(3), 147-164.
Chen, H., et al. (2019). "Advances in biomimetic blood vessel models for studying vascular pathobiology." Biomaterials, 198, 78-94.
Kuang, X., et al. (2021). "Patient-specific 3D-printed blood vessel models for pre-surgical planning and drug testing." Advanced Healthcare Materials, 10(5), 2000806.
Lee, J.H., et al. (2018). "Engineering of vascular-mimetic microfluidic platforms for studying vascular biology and disease models." Progress in Biomedical Engineering, 1(1), 012001.
Wang, Z., et al. (2022). "Microfluidic blood vessel models for studying vascular diseases and drug screening." Lab on a Chip, 22(6), 1077-1093.
Smith, A.S., et al. (2020). "Human iPSC-derived blood vessel organoids for disease modeling and drug testing." Stem Cell Reports, 15(2), 351-365.