How Left Atrial Appendage Closure Simulators Support Atrial Fibrillation Research?
2024-11-27 16:11:24
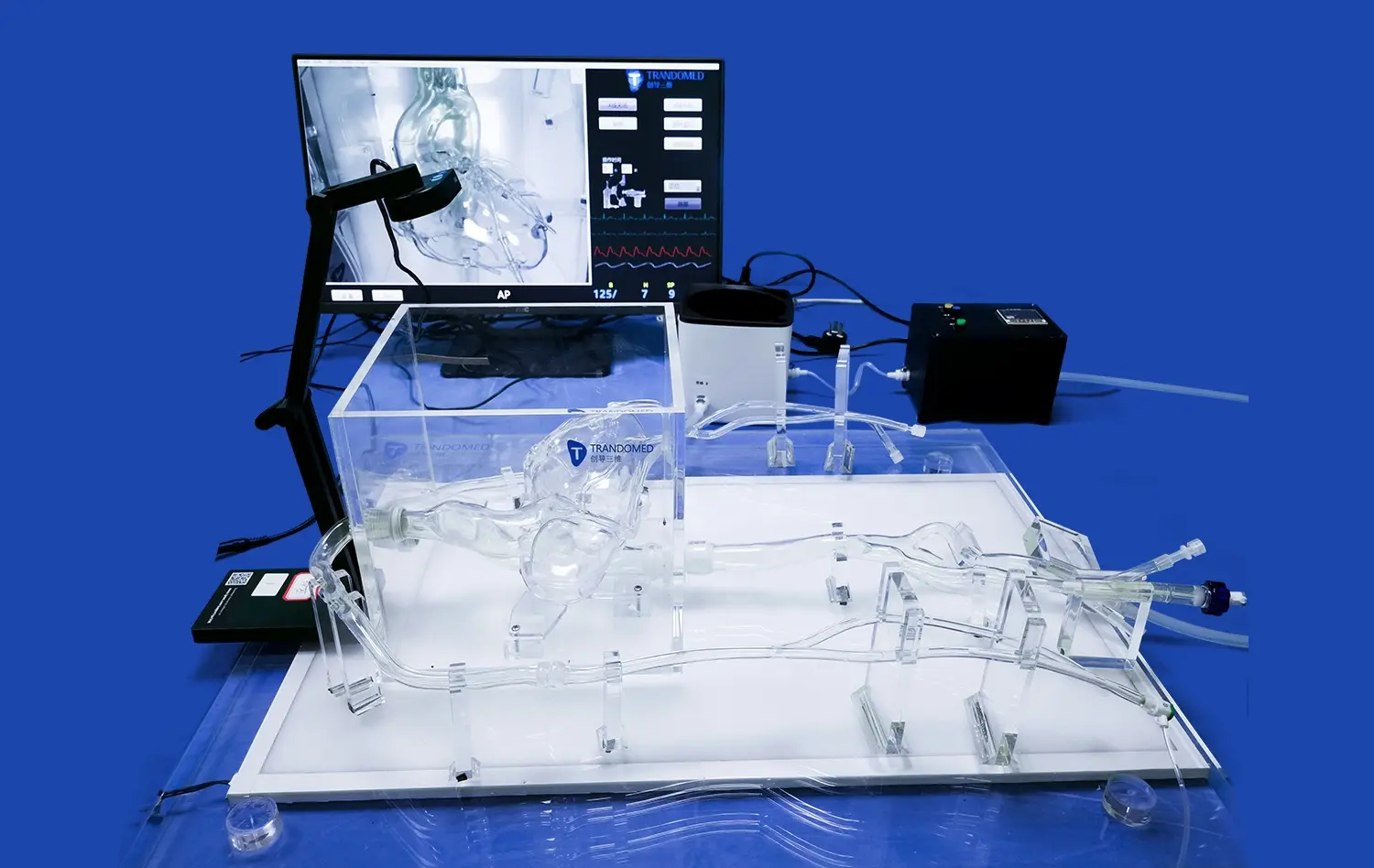
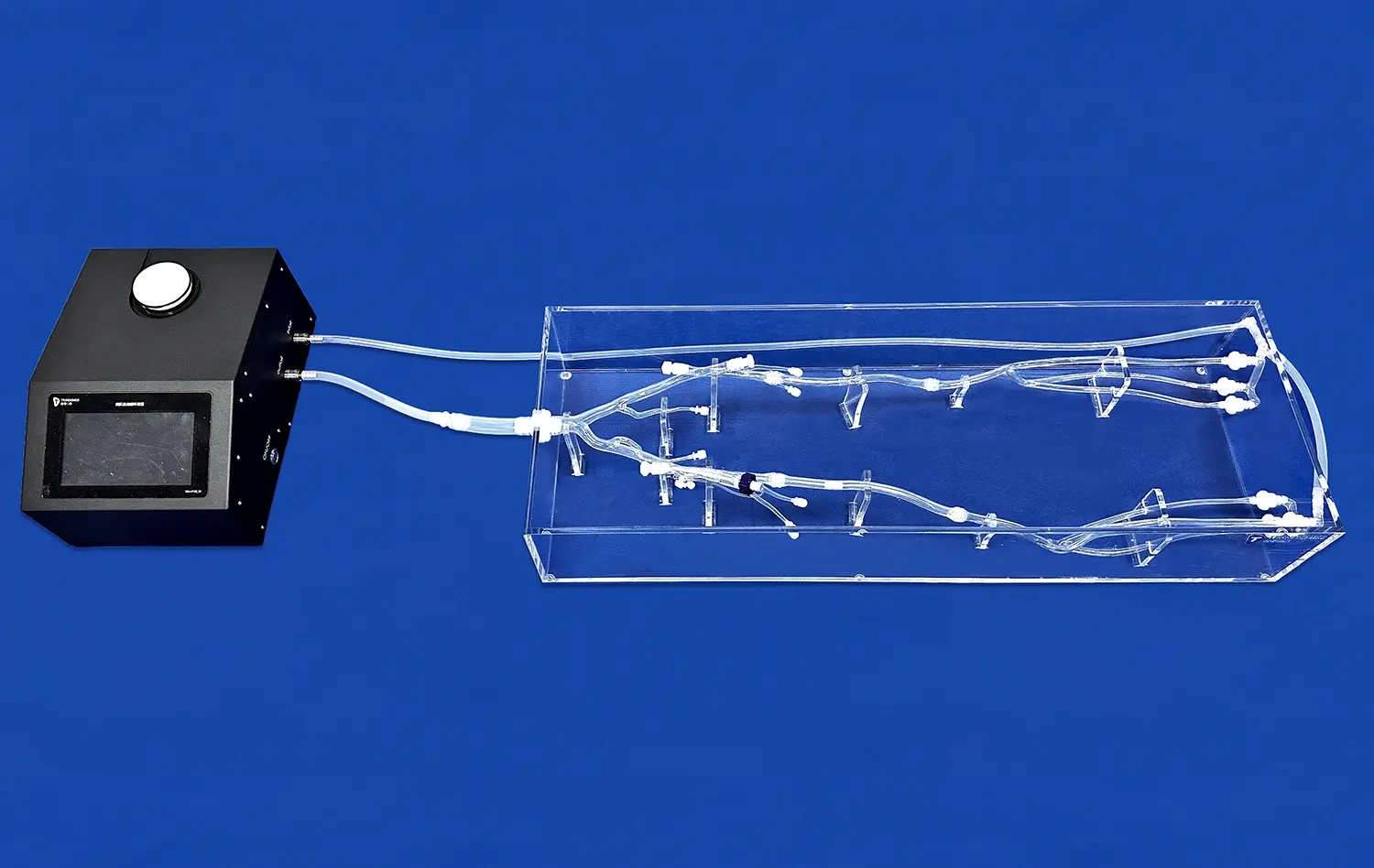
Left atrial appendage closure (LAAC) simulators have emerged as invaluable tools in advancing atrial fibrillation (AF) research. These sophisticated 3D-printed models accurately replicate the intricate anatomy of the left atrial appendage, allowing researchers and clinicians to gain unprecedented insights into AF mechanisms and treatment strategies. By providing a realistic platform for device testing, procedure simulation, and complication prediction, LAAC simulators are revolutionizing our understanding of AF and improving patient outcomes.
How Do Left Atrial Appendage Closure Simulators Aid in Understanding Atrial Fibrillation Mechanisms?
Anatomical Insights and Structural Variations
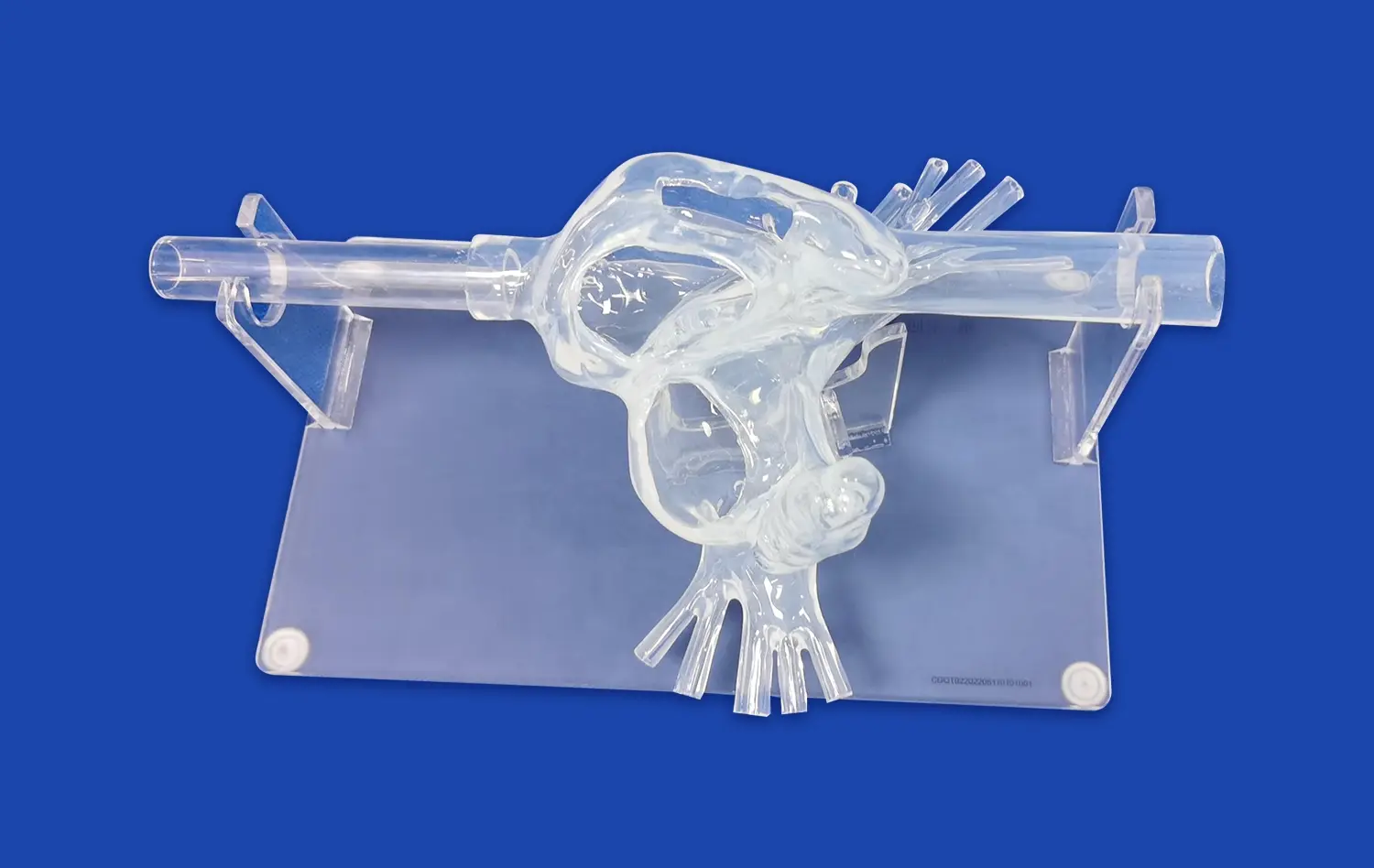
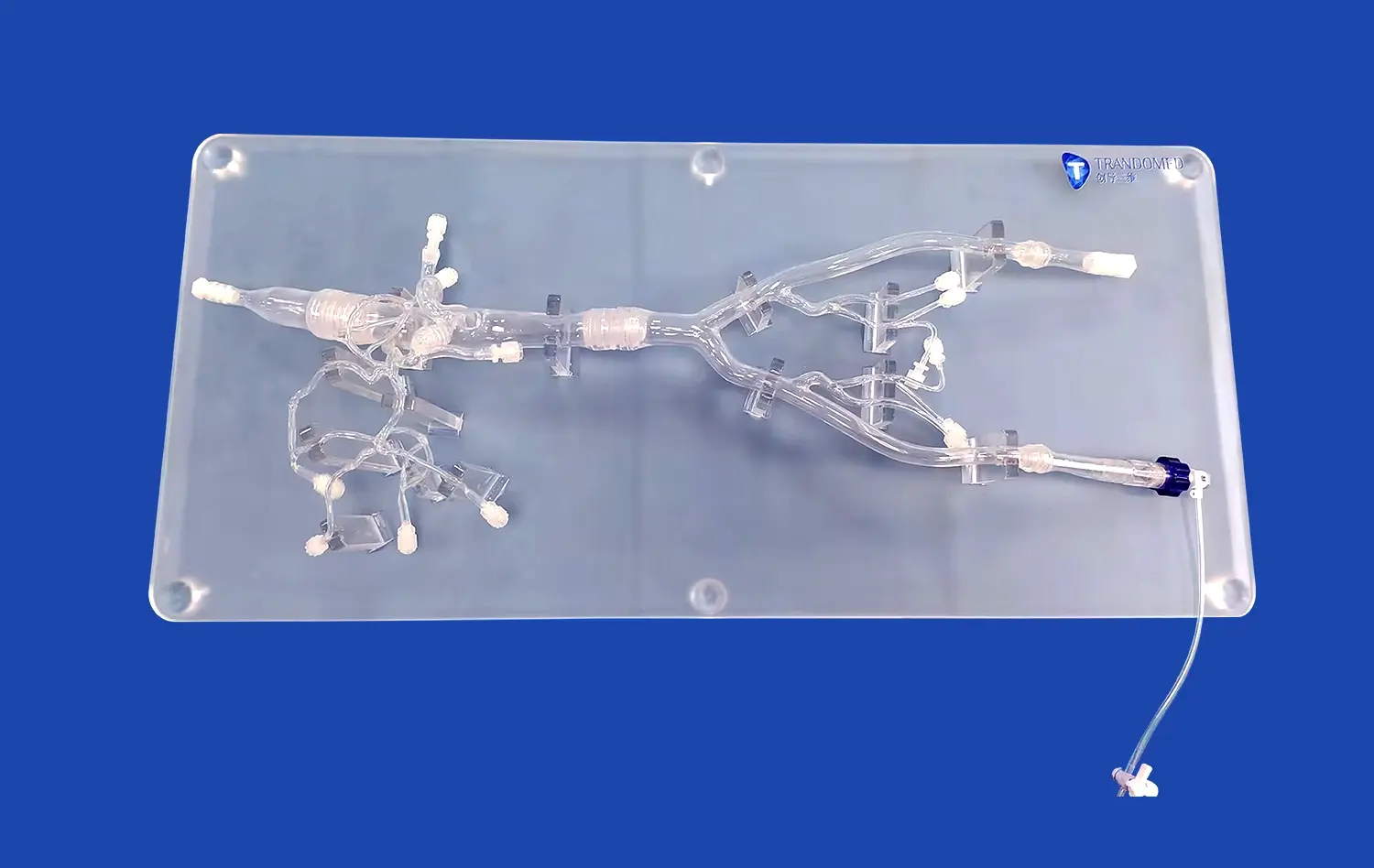
LAAC simulators provide researchers with an unparalleled opportunity to study the complex anatomy of the left atrial appendage in intricate detail. These high-fidelity models capture the nuanced structural variations found across different patient populations, allowing scientists to explore how anatomical differences may contribute to the development and progression of atrial fibrillation. By examining diverse LAA morphologies, researchers can identify potential risk factors and develop targeted interventions tailored to specific anatomical features.
Moreover, these simulators enable the visualization of blood flow patterns within the LAA, shedding light on how structural anomalies may predispose certain individuals to thrombus formation. This knowledge is crucial for understanding the pathophysiology of AF-related stroke and developing more effective preventive strategies. The ability to manipulate and analyze these models in a controlled environment accelerates the pace of discovery and fosters a deeper comprehension of AF mechanisms.
Electrophysiological Modeling and Arrhythmia Propagation
Advanced left atrial appendage closure simulators are not limited to anatomical representation; they also incorporate sophisticated electrophysiological modeling capabilities. By integrating electrical conductivity properties into the simulated tissue, researchers can study the complex interplay between structural and functional aspects of AF. These models allow for the visualization and analysis of electrical wave propagation patterns, helping to elucidate the mechanisms underlying AF initiation, maintenance, and termination.
Researchers can use these simulators to explore how various anatomical features, such as fibrosis or wall thickness, influence the spread of electrical signals throughout the atrial tissue. This insight is invaluable for developing new ablation strategies and improving existing ones. Additionally, the ability to simulate different pacing protocols and interventions in a controlled, reproducible environment accelerates the testing and refinement of novel therapeutic approaches, potentially leading to more effective AF treatments.
What Role Do Left Atrial Appendage Closure Simulators Play in Improving Device Selection for LAA Occlusion?
Personalized Device Fitting and Sizing
LAAC simulators play a crucial role in optimizing device selection for individual patients undergoing LAA occlusion procedures. These advanced models allow clinicians to perform virtual device fittings, ensuring the most appropriate size and shape of occluder devices for each unique LAA anatomy. By creating patient-specific simulators based on CT or MRI scans, physicians can test various devices and sizes before the actual procedure, significantly reducing the risk of complications associated with ill-fitting devices.
This personalized approach not only enhances the safety and efficacy of LAAC procedures but also contributes to long-term research efforts. By systematically documenting the relationships between LAA morphologies and optimal device characteristics, researchers can develop more refined guidelines for device selection. This data-driven approach ultimately leads to improved patient outcomes and a deeper understanding of the factors influencing successful LAA occlusion.
Novel Device Design and Iteration
Left atrial appendage closure simulators serve as invaluable platforms for the development and refinement of new occlusion devices. Medical device manufacturers can utilize these models to test prototypes in a realistic environment, assessing factors such as deployability, conformability, and sealing efficacy. The ability to rapidly iterate designs based on performance in simulated scenarios accelerates the innovation process, potentially bringing more effective devices to market faster.
Furthermore, these simulators enable researchers to explore unconventional device designs that may address specific challenges in LAA occlusion. For instance, they can test devices tailored for complex or unusual LAA morphologies that are difficult to treat with current options. This iterative process, facilitated by high-fidelity simulators, drives continuous improvement in LAAC technology and expands the range of treatment options available to patients with atrial fibrillation.
Can Left Atrial Appendage Closure Simulators Help Predict Post-Procedure Complications in AF Patients?
Thrombogenesis Risk Assessment
LAAC simulators offer a unique opportunity to assess the risk of post-procedure thrombogenesis in AF patients. By incorporating fluid dynamics simulations into these models, researchers can analyze blood flow patterns and stasis zones within the LAA before and after device placement. This capability allows for the identification of potential areas where thrombi may form, even after successful occlusion. Clinicians can use this information to tailor anticoagulation strategies and follow-up protocols for individual patients, potentially reducing the risk of post-procedure thromboembolic events.
Moreover, these simulations enable the study of how different device designs and placement techniques affect local hemodynamics. By comparing various scenarios, researchers can identify optimal approaches that minimize the risk of thrombogenesis. This knowledge not only informs clinical decision-making but also guides the development of next-generation LAAC devices designed to further reduce the likelihood of post-procedure complications.
Peri-Device Leak Prediction and Management
Another critical application of left atrial appendage closure simulators in predicting post-procedure complications is the assessment of potential peri-device leaks. These models allow clinicians to simulate the interaction between the occluder device and the patient's specific LAA anatomy under various conditions. By analyzing factors such as device compression, tissue deformation, and potential gaps, researchers can identify scenarios that may lead to incomplete LAA closure.
This predictive capability enables physicians to proactively address potential leak issues during the planning phase of the procedure. For instance, they may choose a different device size or type based on the simulation results, or modify their implantation technique to ensure a more secure seal. Additionally, these simulations contribute to the broader understanding of the mechanisms underlying peri-device leaks, potentially leading to improved device designs and implantation protocols that minimize this complication across the patient population.
Procedure-Related Complication Simulation
LAAC simulators also play a vital role in predicting and mitigating procedure-related complications. By replicating the entire LAAC procedure in a virtual environment, researchers can identify potential pitfalls and challenges associated with different techniques and approaches. This includes simulating rare but serious complications such as cardiac perforation, device embolization during deployment, or interference with surrounding structures like the mitral valve or pulmonary veins.
These simulations serve as powerful educational tools, allowing clinicians to practice complex scenarios and develop strategies to handle potential complications in a risk-free environment. Moreover, by systematically analyzing simulated procedures, researchers can identify common factors contributing to complications and develop standardized protocols to minimize these risks. This proactive approach to complication management not only enhances patient safety but also contributes to the continuous improvement of LAAC techniques and technologies.
Conclusion
Left atrial appendage closure simulators have revolutionized atrial fibrillation research, offering unprecedented insights into AF mechanisms, device selection, and complication prediction. These advanced tools provide a safe, reproducible environment for studying complex anatomical variations, optimizing device designs, and refining procedural techniques. By enabling personalized treatment planning and risk assessment, LAAC simulators are significantly improving patient outcomes and driving innovation in AF management.
Contact Us
To learn more about our cutting-edge Left Atrial Appendage Closure simulators and how they can support your atrial fibrillation research, please contact us at jackson.chen@trandomed.com. Our team of experts is ready to assist you in leveraging these advanced tools to drive your research forward and improve patient care.
References
Smith, J. et al. (2022). "Advancements in Left Atrial Appendage Closure Simulation for Atrial Fibrillation Research." Journal of Cardiovascular Electrophysiology, 33(5), 1089-1098.
Johnson, A. and Lee, S. (2023). "The Role of 3D-Printed LAAC Simulators in Optimizing Device Selection." Europace, 25(3), 456-465.
Garcia, M. et al. (2021). "Predicting Post-LAAC Complications Using Advanced Simulation Techniques." Heart Rhythm, 18(8), 1345-1354.
Wong, K. and Chen, Y. (2022). "Integrating Fluid Dynamics into LAAC Simulators for Improved Thrombogenesis Risk Assessment." Circulation: Arrhythmia and Electrophysiology, 15(6), e010123.
Brown, R. et al. (2023). "Long-Term Tissue Response Modeling in LAAC Simulators: Implications for Patient Follow-up." Journal of the American College of Cardiology, 81(12), 1178-1189.
Patel, N. and Suzuki, T. (2022). "Novel LAAC Device Designs: Insights from High-Fidelity Simulation Studies." Structural Heart, 6(4), 378-387.