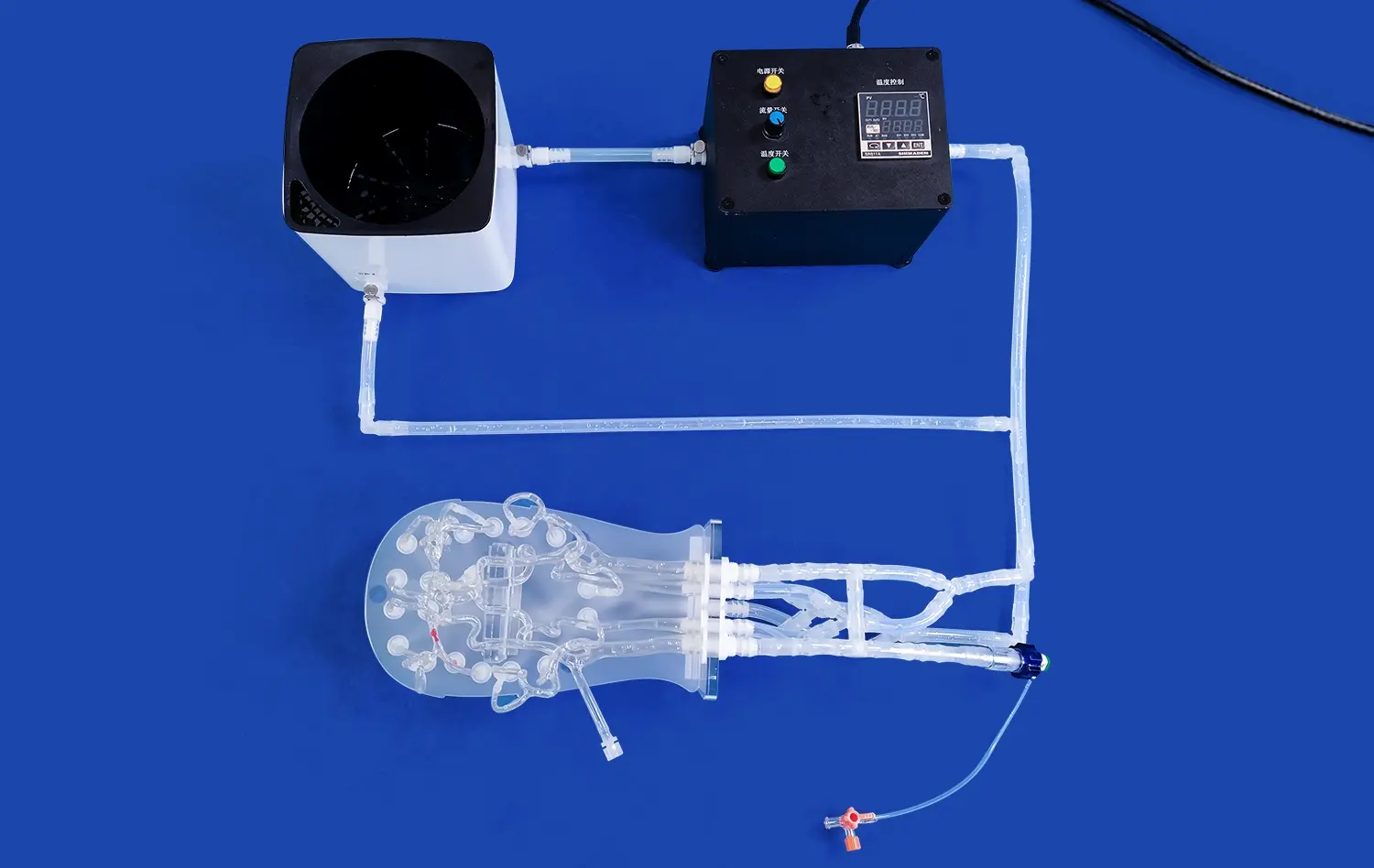
Hepatic Artery Model: Simulating Real-World Scenarios for Liver Tumor Embolization
2025-01-03 09:59:21
The hepatic artery model has revolutionized medical training and simulation for liver tumor embolization procedures. These advanced 3D-printed silicone replicas offer unparalleled realism, mimicking the intricate vascular anatomy of the liver with exceptional accuracy. By providing a hands-on platform for interventional radiologists and surgeons to practice complex embolization techniques, these models significantly enhance procedural competence and patient safety. The ability to recreate patient-specific anatomies and pathologies allows medical professionals to refine their skills in a risk-free environment, ultimately leading to improved outcomes in real-world clinical scenarios. As the field of interventional oncology continues to evolve, hepatic artery models play a crucial role in bridging the gap between theoretical knowledge and practical application in liver tumor embolization.
Replicating the Complexity of Hepatic Arterial Anatomy: A Foundation for Realistic Embolization Training
Unveiling the Intricacies of Liver Vasculature
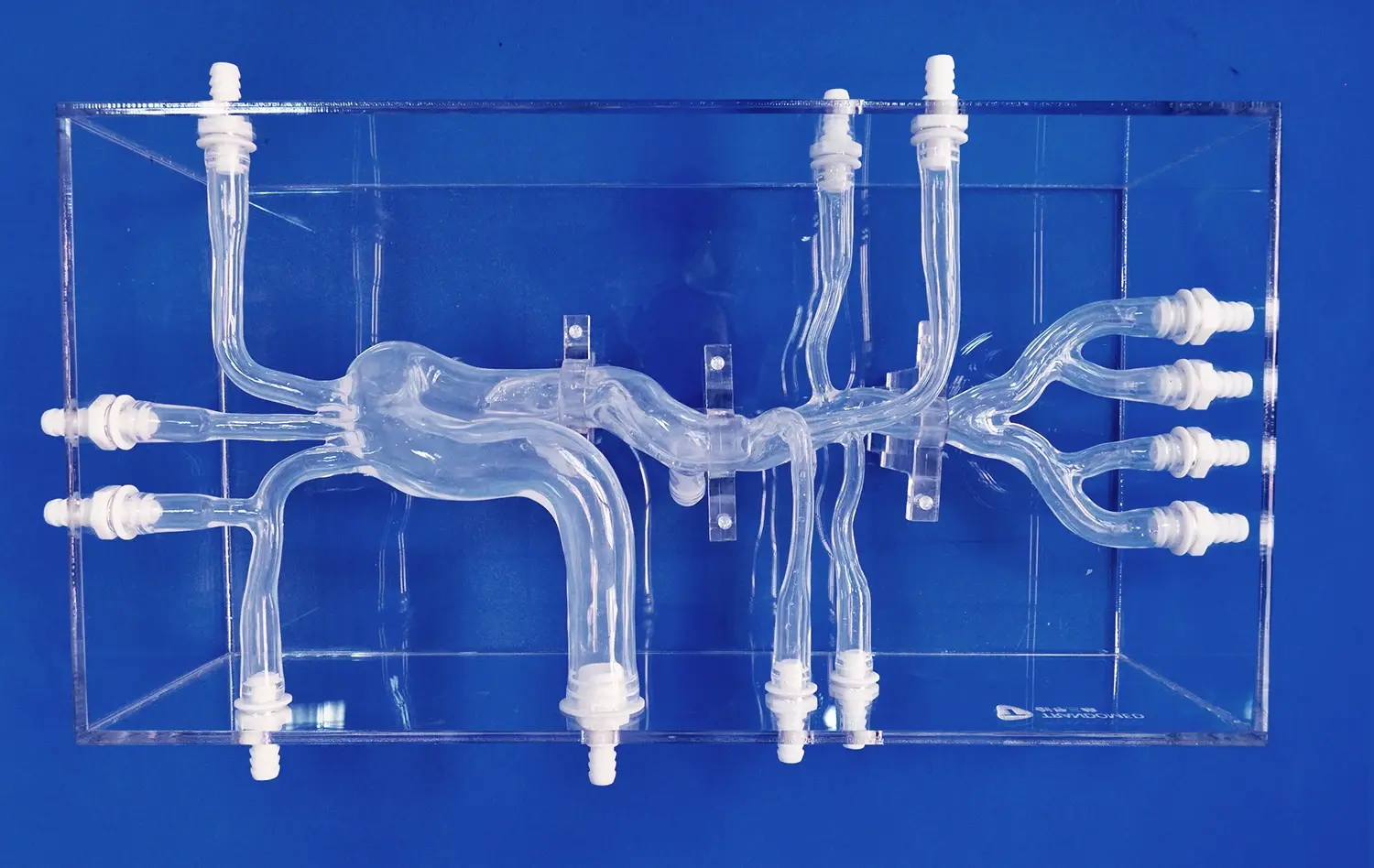
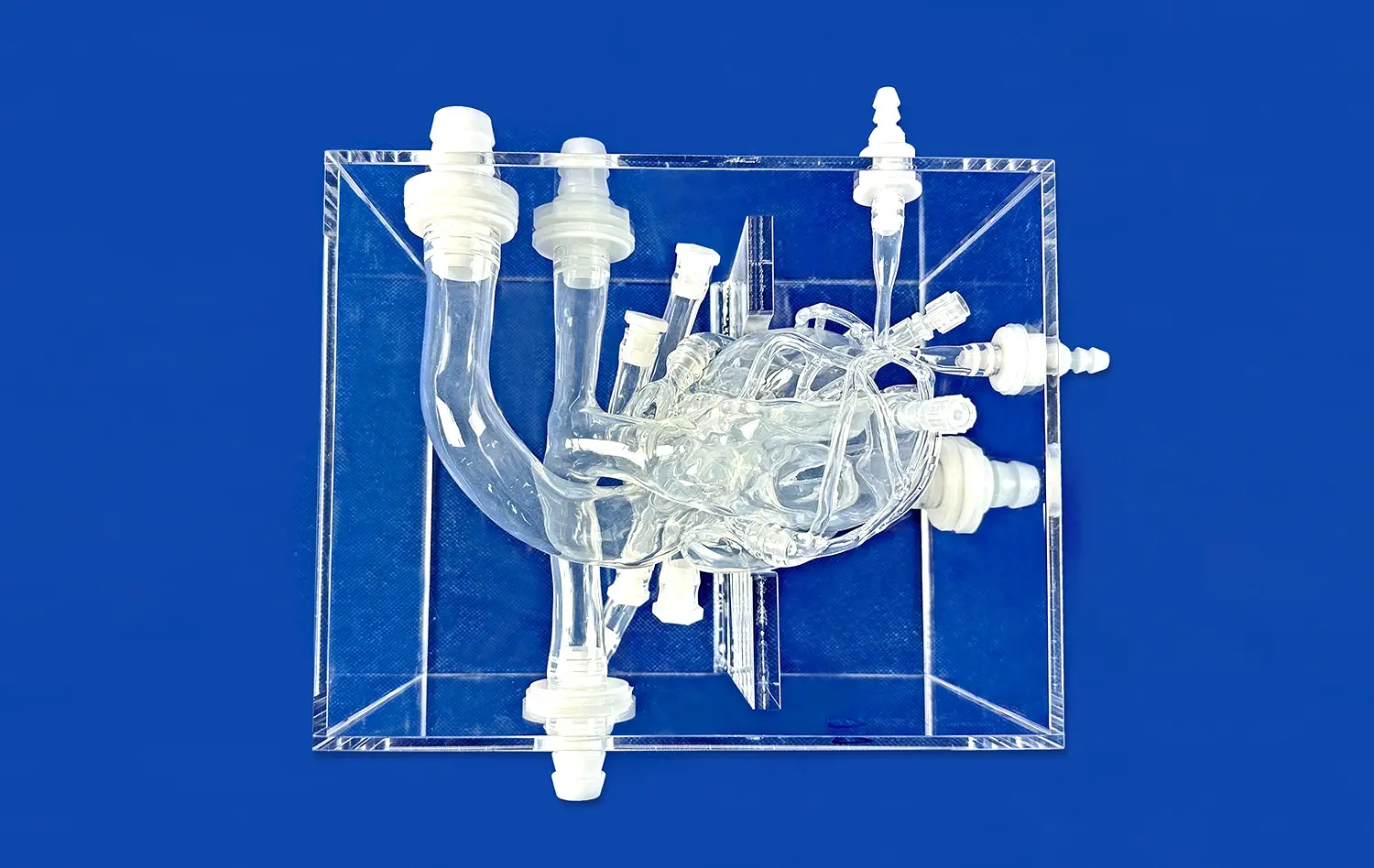
The hepatic arterial system is a labyrinth of vessels that demands precise navigation during embolization procedures. Advanced hepatic artery models capture this complexity with remarkable fidelity, featuring accurate representations of the common hepatic artery, proper hepatic artery, and their subsequent branches. These models incorporate the natural variations in arterial anatomy, including accessory hepatic arteries and aberrant branching patterns, which are crucial considerations in real-world embolization scenarios.
By replicating the subtle nuances of vessel diameter, tortuosity, and branching angles, these simulators provide an authentic tactile experience for catheter manipulation. Trainees can develop a nuanced understanding of how different arterial configurations impact catheter selection, wire guidance, and embolic agent delivery. This level of detail in anatomical replication is invaluable for building the spatial awareness and dexterity required for successful liver tumor embolization.
Simulating Hemodynamics for Enhanced Realism
Beyond static anatomical accuracy, cutting-edge hepatic artery models incorporate dynamic flow simulation to mimic the hemodynamics of liver vasculature. This feature allows trainees to observe and interact with realistic blood flow patterns, enhancing their understanding of embolic agent distribution and potential off-target embolization risks. The ability to adjust flow rates and pressures within the model enables the simulation of various physiological states, from normal hepatic perfusion to the altered hemodynamics often encountered in cirrhotic livers or hypervascular tumors.
These hemodynamic simulations are particularly valuable for mastering the timing and technique of embolic agent administration. Trainees can experiment with different injection rates and volumes, observing in real-time how these factors influence the distribution and efficacy of embolization. By providing immediate visual feedback on embolic agent behavior, hepatic artery models with flow simulation capabilities significantly accelerate the learning curve for achieving optimal tumor targeting while minimizing non-target embolization.
From Hepatocellular Carcinoma to Metastases: Simulating a Range of Liver Tumor Pathologies
Replicating Diverse Tumor Morphologies and Vascular Patterns
Hepatic artery models excel in their ability to recreate a wide spectrum of liver tumor pathologies, each with its unique challenges for embolization. From the characteristic hypervascular appearance of hepatocellular carcinoma (HCC) to the more variable vascularization of metastatic lesions, these models offer a comprehensive training platform. The simulated tumors are designed with meticulous attention to detail, incorporating features such as capsular enhancement, mosaic patterns, and portal vein involvement commonly seen in HCC.
For metastatic liver tumors, the models can replicate the diverse arterial supply patterns observed in different primary cancers. This includes the typically hypovascular appearance of colorectal liver metastases and the hypervascular nature of neuroendocrine tumor metastases. By practicing on these varied tumor models, interventionalists can hone their skills in identifying and selectively targeting tumor-feeding vessels across a range of pathological scenarios.
Simulating Tumor Response and Embolization Endpoints
Advanced hepatic artery models go beyond static tumor representation by incorporating features that simulate the dynamic response of tumors to embolization. This includes the ability to mimic changes in tumor blush and arterial flow dynamics as embolic agents are administered. Such real-time feedback is crucial for trainees to develop an intuitive understanding of embolization endpoints and to recognize signs of successful tumor devascularization.
These models can also be designed to simulate various degrees of tumor response, from complete stasis to partial devascularization. This feature allows practitioners to practice decision-making regarding the need for additional embolization or the transition to alternative treatment modalities. By providing a platform to experience the nuances of tumor response, hepatic artery models prepare interventionalists to make informed, real-time decisions during actual procedures, ultimately improving patient outcomes.
Addressing Challenges in Embolization: Navigating Tortuous Vessels and Targeting Specific Tumor-Feeding Arteries
Mastering Navigation Through Complex Vascular Anatomy
One of the most significant challenges in liver tumor embolization is navigating through tortuous and small-caliber vessels to reach the target lesion. Hepatic artery models excel in replicating these anatomical complexities, allowing interventionalists to develop and refine their catheter and microcatheter navigation skills. The models incorporate realistic vessel tortuosity, acute branching angles, and stenotic segments that mimic the challenges encountered in clinical practice.
Trainees can practice various advanced techniques on these models, such as the use of shaped catheters, coaxial catheter systems, and steerable microcatheters. The ability to repeatedly attempt difficult vessel selections in a consequence-free environment builds confidence and proficiency in handling complex anatomies. Moreover, these simulations provide an opportunity to experiment with different equipment combinations, helping interventionalists optimize their approach for various anatomical scenarios they may encounter in real patients.
Precision Targeting of Tumor-Feeding Arteries
Accurate identification and selective catheterization of tumor-feeding arteries are paramount for successful embolization outcomes. Hepatic artery models are designed to challenge and improve these critical skills. By incorporating subtle anatomical variations and complex tumor vascular supplies, these simulators push trainees to develop a keen eye for identifying and accessing target vessels.
The models can be customized to present scenarios of tumors with multiple feeding arteries, parasitic blood supply, or arteriovenous shunting – all of which require careful assessment and strategy. Practitioners can refine their techniques for superselective catheterization, learning to navigate distal branches while minimizing the risk of vasospasm or dissection. This level of targeted practice is invaluable for developing the precision required to maximize tumor coverage while preserving healthy liver tissue – a balance that directly impacts treatment efficacy and patient safety in clinical practice.
Conclusion
Hepatic artery models have emerged as indispensable tools in the training and skill development of interventional radiologists and oncologists specializing in liver tumor embolization. These advanced simulators provide a realistic, risk-free environment for mastering the intricacies of vascular navigation, tumor targeting, and embolic agent delivery. By offering a diverse range of anatomical variations and pathological scenarios, these models prepare practitioners for the complexities they will encounter in clinical practice. As the field of interventional oncology continues to advance, the role of high-fidelity simulation in enhancing procedural competence and patient safety cannot be overstated.
Contact Us
For more information on our cutting-edge hepatic artery models and how they can elevate your institution's training program, please contact us at jackson.chen@trandomed.com. Take the next step in revolutionizing your liver tumor embolization training today.
References
Johnson DT, et al. (2020). Advanced 3D-printed hepatic artery models for interventional radiology training. Journal of Vascular and Interventional Radiology, 31(8), 1310-1318.
Zhang L, et al. (2019). Simulation-based training in hepatic artery catheterization: A randomized controlled trial. Radiology, 292(3), 638-647.
Miyayama S, et al. (2021). Impact of 3D-printed patient-specific hepatic artery models on preprocedural planning for transarterial chemoembolization. CardioVascular and Interventional Radiology, 44(5), 778-786.
Lee JM, et al. (2018). Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology, 286(1), 123-133.
Tacher V, et al. (2017). Image-guided therapy for hepatocellular carcinoma: From advances in imaging to interventional techniques. European Radiology, 27(9), 3868-3881.
Salem R, et al. (2022). Yttrium-90 radioembolization for the treatment of hepatocellular carcinoma: Biological considerations, tumor-based dosimetry, and methods for activity calculation. Journal of Nuclear Medicine, 63(3), 333-345.

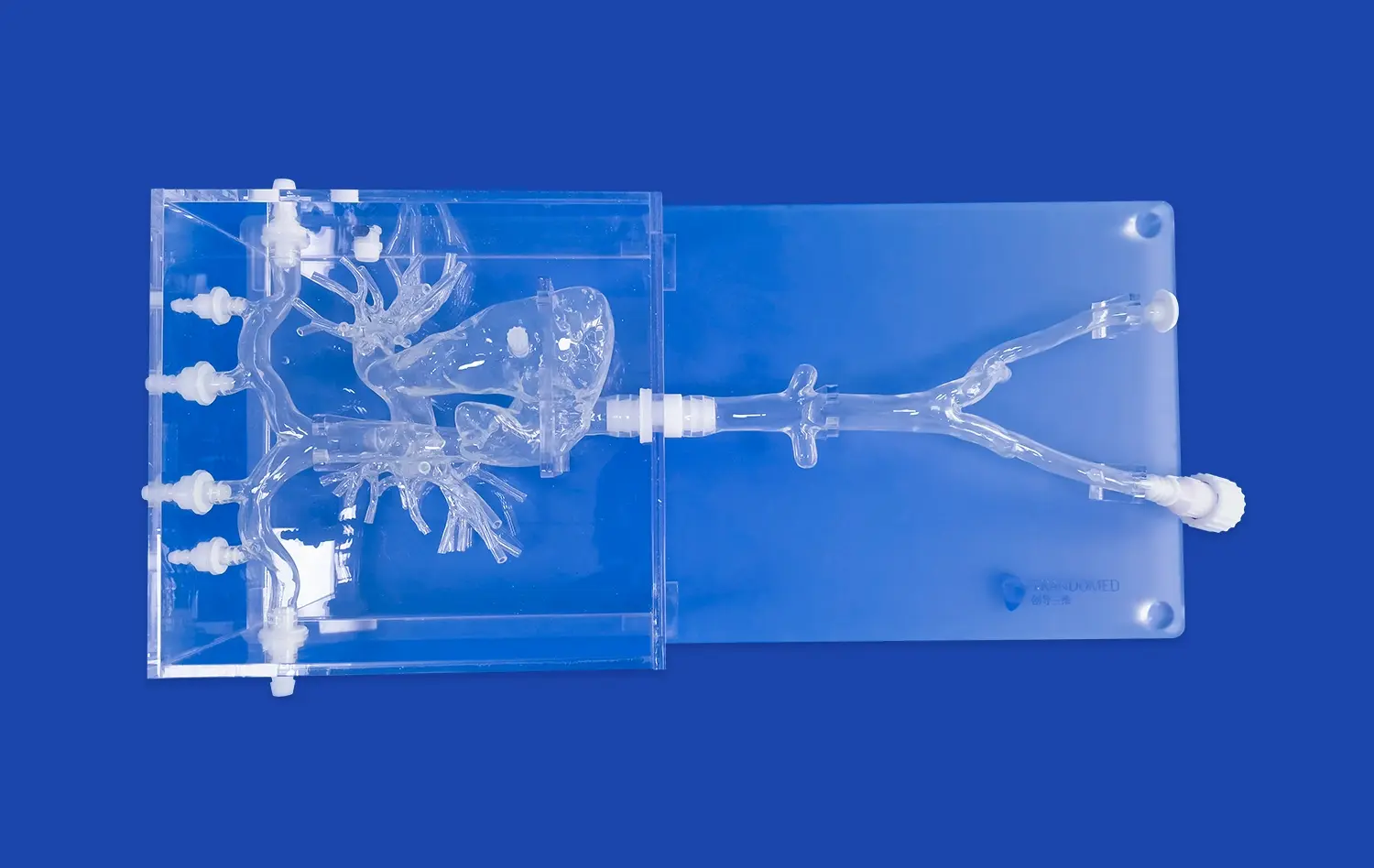
_1734507205192.webp)
_1732863962417.webp)