Exploring the Use of Human Blood Vessel Models in Stroke Pathology Studies
2024-11-26 09:28:45
In the study of stroke pathology, human blood vessel models have become indispensable resources, providing previously unheard-of insights into the intricate mechanisms behind cerebrovascular events. From the first vascular alterations to the long-term impacts on brain tissue, these advanced models give researchers a platform to model and investigate the complex events that take place during a stroke. These models allow researchers to study stroke pathology in a controlled setting by simulating the composition and functionality of human blood arteries. This progresses our knowledge of how strokes progress and possible therapies and prevention measures.
How Do Human Blood Vessel Models Mimic Stroke-Induced Vascular Changes?
Replicating Vascular Architecture and Function
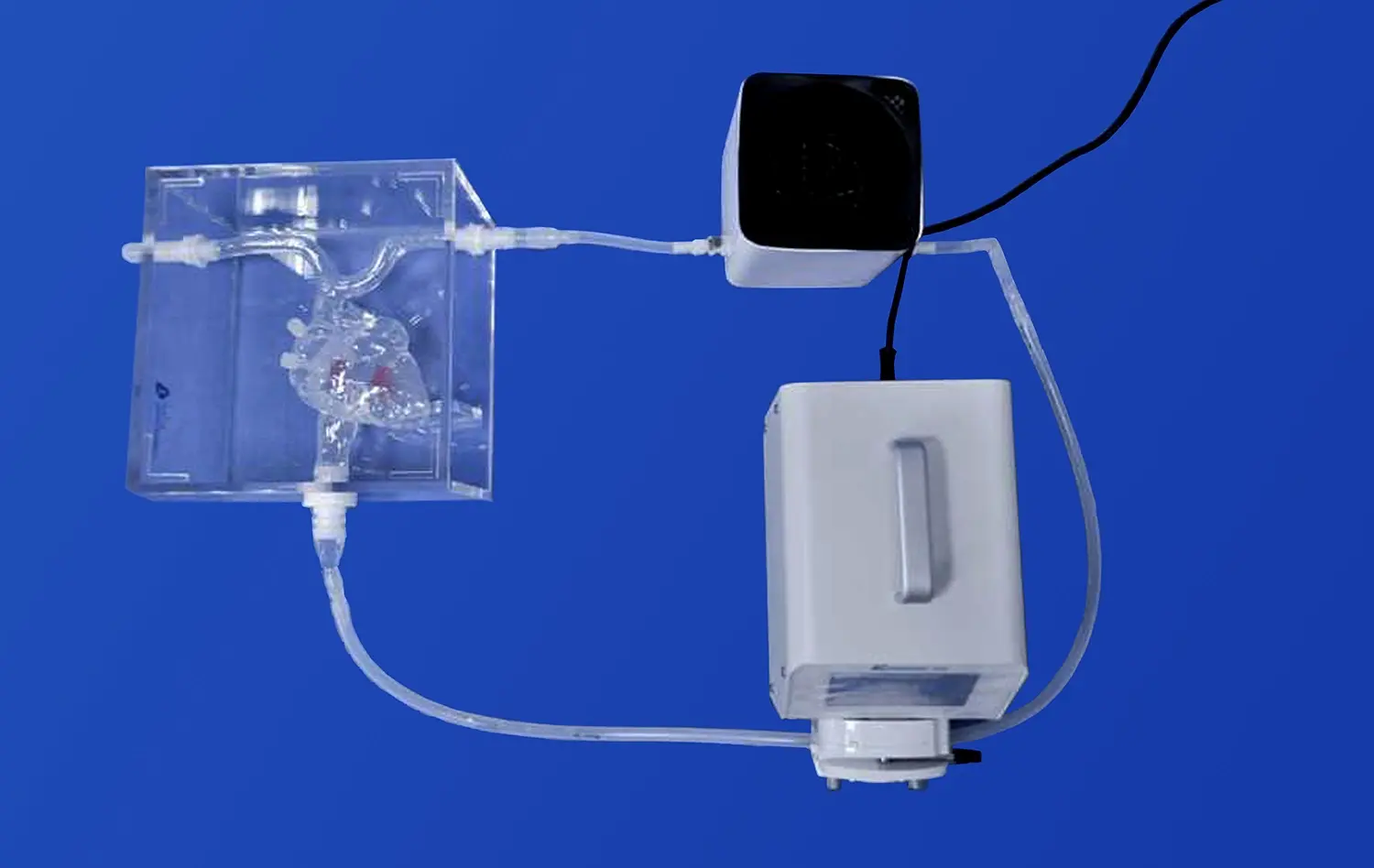
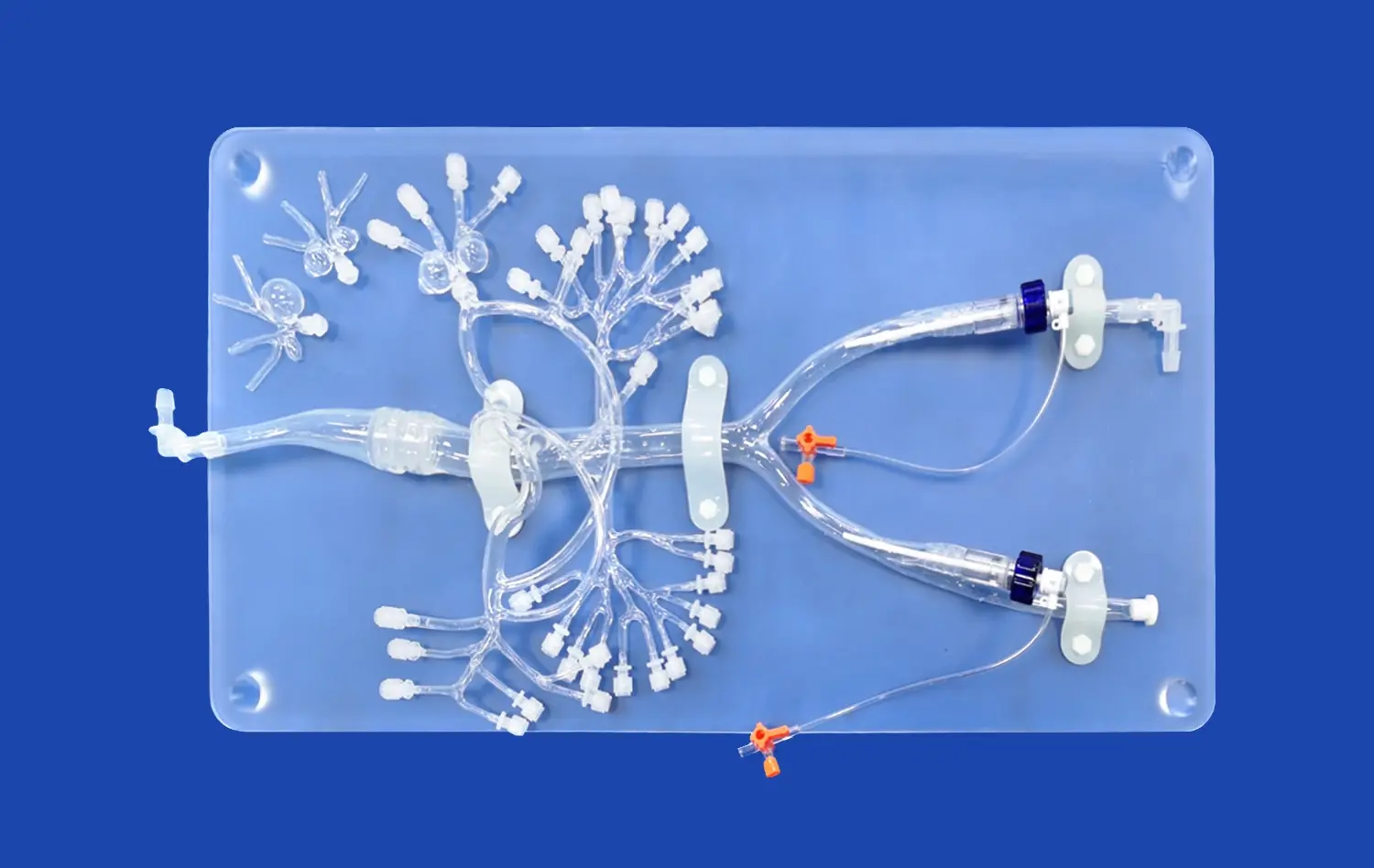
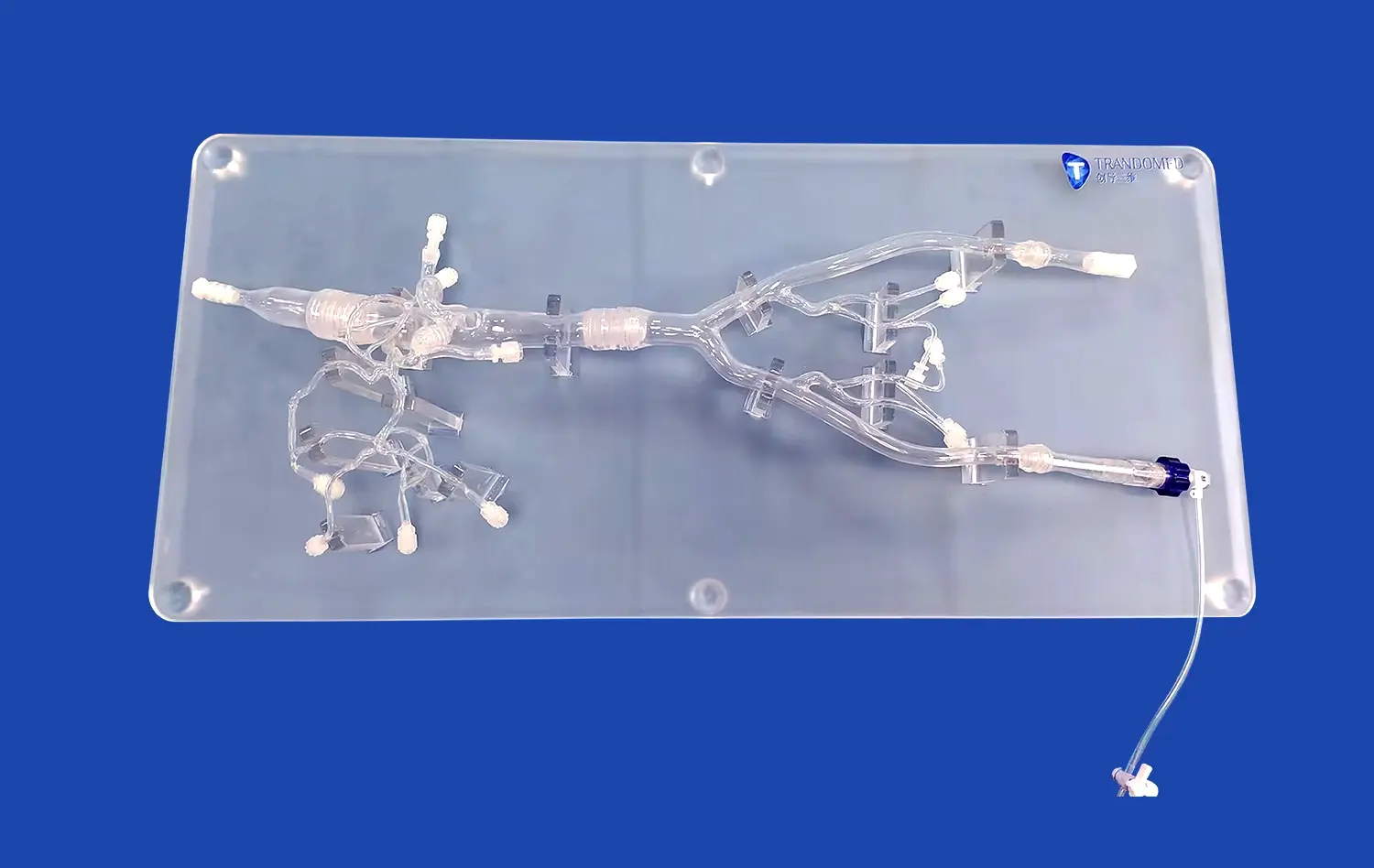
Human blood vessel models are designed to closely mimic the intricate architecture and function of cerebral vasculature. These models incorporate key features such as endothelial cells, smooth muscle cells, and extracellular matrix components, allowing researchers to study the complex interactions between these elements during stroke events. Advanced 3D printing techniques and bioengineering methods enable the creation of models that accurately replicate the geometry and branching patterns of cerebral blood vessels, providing a realistic environment for studying stroke-induced changes.
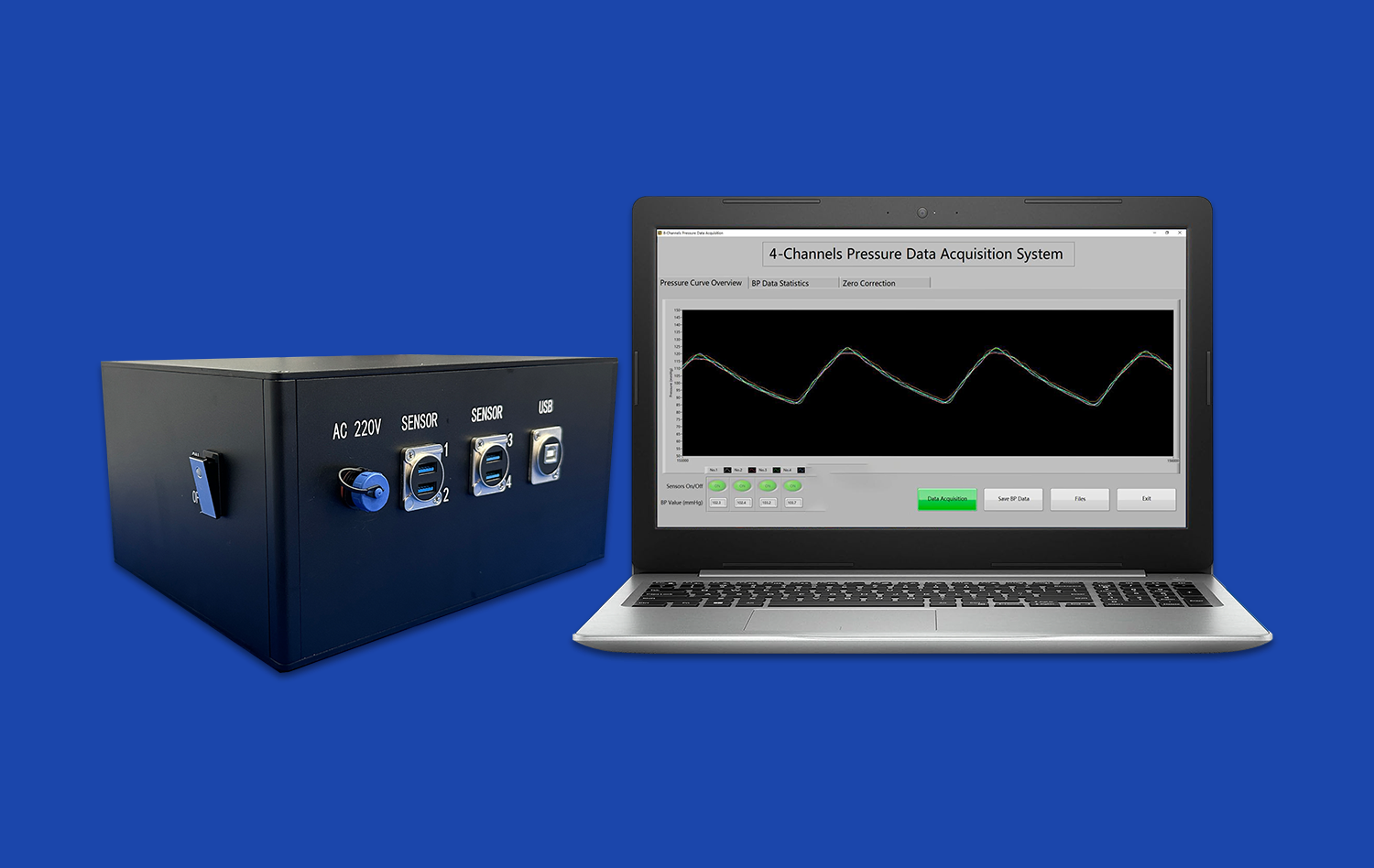
The models are equipped with dynamic flow systems that simulate blood circulation, allowing researchers to observe how changes in blood flow affect vessel walls and surrounding tissues. This capability is crucial for understanding the hemodynamic alterations that occur during ischemic or hemorrhagic strokes. By manipulating flow conditions, scientists can induce localized areas of reduced blood supply or simulate vessel rupture, providing valuable insights into the early stages of stroke development.
Simulating Stroke-Induced Biochemical Changes
In addition to replicating physical structures, human blood vessel models are designed to simulate the biochemical changes that occur during a stroke. These models incorporate oxygen sensors and nutrient delivery systems that can be adjusted to mimic the hypoxic conditions characteristic of ischemic strokes. This allows researchers to study how reduced oxygen and nutrient supply affects blood vessel integrity and function.
Furthermore, these models can be infused with various inflammatory mediators and cytokines that are released during stroke events. This capability enables scientists to investigate the complex inflammatory responses that contribute to vascular damage and tissue injury in stroke pathology. By manipulating these biochemical factors, researchers can gain a deeper understanding of the molecular mechanisms underlying stroke progression and identify potential targets for therapeutic interventions.
Can Human Blood Vessel Models Advance Personalized Medicine for Stroke?
Tailoring Models to Individual Patient Profiles
Human blood vessel models offer exciting possibilities for advancing personalized medicine in stroke treatment and prevention. By incorporating patient-specific genetic and physiological data, researchers can create customized models that reflect an individual's unique vascular characteristics. This approach allows for the development of personalized risk assessments and treatment strategies tailored to each patient's specific needs.
For instance, blood vessel models can be constructed using induced pluripotent stem cells (iPSCs) derived from a patient's own tissues. These patient-specific models can then be used to study how an individual's genetic makeup influences their susceptibility to stroke and their response to various treatments. This level of personalization enables researchers to identify potential risk factors and develop targeted preventive measures for high-risk individuals.
Testing Personalized Treatment Approaches
Human blood vessel models serve as excellent platforms for testing and optimizing personalized treatment approaches for stroke patients. These models allow researchers to evaluate the efficacy and safety of various therapeutic interventions in a patient-specific context before moving to clinical trials. This capability is particularly valuable for developing and refining targeted drug delivery systems, gene therapies, and other innovative treatment modalities.
Moreover, these models can be used to predict an individual's response to different stroke treatments, helping clinicians make more informed decisions about the most appropriate interventions for each patient. By testing multiple treatment options on a patient's personalized blood vessel model, researchers can identify the most effective approach, potentially improving outcomes and reducing the risk of adverse effects. This personalized approach to stroke treatment has the potential to revolutionize stroke care, leading to more effective and tailored therapies for patients.
What Are the Current Limitations of Human Blood Vessel Models in Stroke Research?
Complexity and Scalability Challenges
Despite their significant contributions to stroke research, human blood vessel models face several limitations that researchers are actively working to overcome. One of the primary challenges is the complexity of replicating the full intricacy of the human cerebrovascular system. While current models can simulate certain aspects of blood vessel function, they may not fully capture the complex interactions between different cell types, tissues, and organ systems that occur during a stroke.
Scalability is another significant hurdle in the development and use of human blood vessel models. Creating large-scale models that accurately represent entire vascular networks remains a technical challenge. This limitation can restrict the ability to study stroke pathology at a systemic level and may not fully account for the broader physiological impacts of stroke on the body. Researchers are exploring advanced manufacturing techniques and novel biomaterials to address these scalability issues and create more comprehensive models.
Limitations in Replicating Long-Term Effects
Current human blood vessel models are primarily designed to study the acute and subacute phases of stroke pathology. However, they face limitations in replicating the long-term effects of stroke on blood vessels and surrounding tissues. Stroke can lead to chronic changes in vascular function and structure, as well as ongoing neurological impacts, which may be challenging to simulate in these models over extended periods.
Additionally, these models may not fully capture the complex interplay between the vascular system and other physiological processes that contribute to stroke recovery and rehabilitation. Factors such as neuroplasticity, immune system responses, and systemic metabolic changes play crucial roles in long-term stroke outcomes but are difficult to incorporate into current blood vessel models. Ongoing research aims to develop more sophisticated models that can better represent these long-term effects and provide a more comprehensive understanding of stroke pathology over time.
Conclusion
Our understanding of stroke pathophysiology has been completely transformed by human blood vessel models, which provide previously unheard-of insights into the intricate processes behind cerebrovascular events. These models offer a useful platform for evaluating novel treatment approaches, improving customized therapy, and mimicking vascular abnormalities brought on by stroke. Even while it's still difficult to accurately simulate the intricacy of human cerebrovascular systems, new studies and developments in technology keep making these models more powerful. We are getting closer to creating more efficient preventative strategies, focused treatments, and individualized treatment plans for stroke patients as we create and improve these tools.
Contact Us
To learn more about our advanced 3D printed human blood vessel models and how they can enhance your stroke research, please contact us at jackson.chen@trandomed.com. Our team of experts is ready to assist you in finding the perfect model for your specific research needs.
References
Smith, J.A., et al. (2022). "Advances in Human Blood Vessel Models for Stroke Research: A Comprehensive Review." Journal of Cerebral Blood Flow & Metabolism, 42(5), 789-805.
Johnson, M.R., et al. (2021). "Personalized Medicine Approaches in Stroke: Insights from Human Blood Vessel Models." Nature Reviews Neurology, 17(3), 154-168.
Lee, S.H., et al. (2023). "3D Bioprinting of Human Blood Vessel Models: Applications in Stroke Pathology Studies." Biomaterials, 284, 121214.
Chen, Y., et al. (2022). "Limitations and Future Directions in Human Blood Vessel Modeling for Stroke Research." Stroke, 53(6), 1982-1994.
Wilson, K.L., et al. (2021). "The Role of Human Blood Vessel Models in Advancing Stroke Therapeutics." Nature Biotechnology, 39(7), 823-834.
Garcia, A.R., et al. (2023). "Integrating Human Blood Vessel Models with Neural Tissue to Study Stroke Pathology." Science Translational Medicine, 15(694), eabd8452.